

One day Courtois added too much sulfuric acid and a cloud of purple vapor rose. The remaining waste was destroyed by adding sulfuric acid.
To isolate the sodium carbonate, seaweed was burned and the ash then washed with water. Saltpeter produced from French niter beds required sodium carbonate, which could be isolated from seaweed washed up on the coasts of Normandy and Brittany. At the time of the Napoleonic Wars, France was at war and saltpeter was in great demand.

He was born to a manufacturer of saltpeter (a vital part of gunpowder). Iodine was discovered by Bernard Courtois in 1811. In fact, if iodine crystals are heated carefully to just above their melting point of 113.7 ☌, the crystals melt into a liquid which is present under a dense blanket of the vapor. This misconception arises because the small amount of vapor produced has such a deep colour that the liquid appears not to form. Students who have seen the classroom demonstration in which iodine crystals are gently heated in a test tube to violet vapor, may gain the impression that liquid iodine does not exist at atmospheric pressure.
USES OF IODINE AS ELEMENT FREE
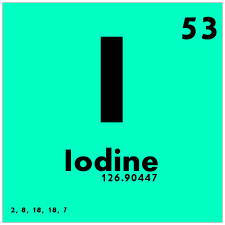
The deep blue color of starch-iodine complexes is produced only by the free element. This is also the formulation of some types of medicinal (antiseptic) iodine, although tincture of iodine classically dissolves the element in alcohol. The molecular iodine reacts reversibly with the negative ion, creating the triiodide anion, I 3 −, which dissolves well in water. The solubility of elementary iodine in water can be vastly increased by the addition of potassium iodide. This halogen forms compounds with many elements, but is less reactive than the other members of its Group VII (halogens) and has some metallic light reflectance.Įlemental iodine dissolves easily in chloroform and carbon tetrachloride. It can be seen apparently subliming at standard temperatures into a violet-pink gas that has an irritating odor. Iodine under standard conditions is a dark-purple/dark-brown solid. This mechanism helps to explain how the element came to be required in trace amounts by all animals and some plants, being by far the heaviest element known to be necessary to living organisms. Although it is rare in the solar system and Earth's crust, the iodides are very soluble in water, and the element is concentrated in seawater. Iodine and its compounds are primarily used in medicine, photography and in dyes. As with all other halogens (members of Group VII in the Periodic Table), when freed from its compounds iodine forms diatomic molecules ( I 2). However, the element does not occur in the free state in nature. Naturally-occurring iodine is a single isotope with 74 neutrons.Ĭhemically, iodine is the least reactive of the halogens, and the most electropositive halogen after astatine. Iodine ( Template:IPAEng, or Template:IPA from Template:Lang-el "violet"), is a chemical element that has the symbol I and atomic number 53. Template:Elementbox isotopes stable Template:Elementbox isotopes decay Template:Elementbox isotopes decay Template:Elementbox isotopes end Template:Elementbox footer ! colspan="2" style="background:#ffff99 color:black" | Selected isotopes
USES OF IODINE AS ELEMENT SERIES
Template:Elementbox header Template:Elementbox series Template:Elementbox groupperiodblock Template:Elementbox appearance img Template:Elementbox atomicmass gpm Template:Elementbox econfig Template:Elementbox epershell Template:Elementbox section physicalprop Template:Elementbox phase Template:Elementbox density gpcm3nrt Template:Elementbox meltingpoint Template:Elementbox boilingpoint Template:Elementbox criticalpoint Template:Elementbox heatfusion kjpmol Template:Elementbox heatvaporiz kjpmol Template:Elementbox heatcapacity jpmolkat25 Template:Elementbox vaporpressure katpa Template:Elementbox section atomicprop Template:Elementbox crystalstruct Template:Elementbox oxistates Template:Elementbox electroneg pauling Template:Elementbox ionizationenergies3 Template:Elementbox atomicradius pm Template:Elementbox atomicradiuscalc pm Template:Elementbox covalentradius pm Template:Elementbox vanderwaalsrad pm Template:Elementbox section miscellaneous Template:Elementbox magnetic Template:Elementbox eresist ohmmat0 Template:Elementbox thermalcond wpmkat300k Template:Elementbox bulkmodulus gpa Template:Elementbox cas number


 0 kommentar(er)
0 kommentar(er)
